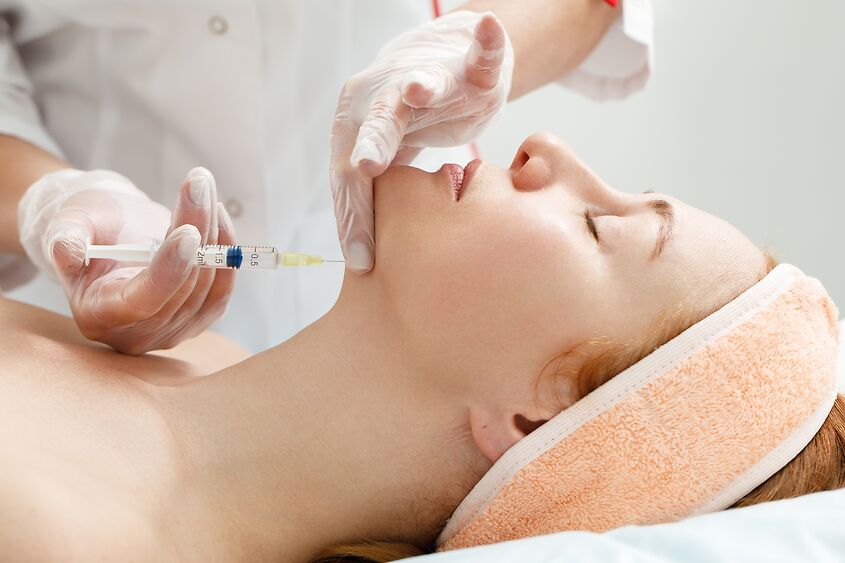
Tapencarium, a groundbreaking nonsurgical injectable solution for permanent fat reduction, stands as a remarkable departure from traditional surgical interventions. Despite being in the early phases of FDA clinical trials, the medical community is already expressing optimism about Tapencarium's potency and effectiveness, anticipating its transformative impact on the landscape of modern surgical practices. Embark on an enlightening journey with renowned Haute Beauty Expert, Dr. Sachin Shridharani, as he unravels the mysteries of Tapencarium and addresses all your inquiries.
How does Tapencarium differ from traditional surgical solutions, and what makes it potentially revolutionary?
Tapencarium differs from traditional surgical solutions because it is a nonsurgical injectable solution for permanent fat reduction. While Kybella/Belkyra, an FDA-approved fat-destroying injectable, holds a presence in the market, Tapencarium distinguishes itself by showcasing clinical efficacy in the permanent destruction of fat within a single session during phase 1 and phase 2 FDA clinical trials. This stands in stark contrast to other injectable treatments, which necessitate multiple sessions for comparable results.
What scientific advancements or breakthroughs contributed to the development of Tapencarium as a syringe-based surgical solution?
The primary advancement is that Tapencarium is a non-animal, non-human derived synthetic molecule that permanently destroys fat cells when it comes into contact with them.
View this post on Instagram
In what medical procedures could Tapencarium be particularly beneficial, and why?
Tapencarium's investigative focus has extended to the targeted destruction of fat cells in areas such as the double chin region and flanks, alongside its exploration in conjunction with the rare condition Dercums disease (adiposis dolorosa). This affliction is marked by the presence of multiple painful growths of fatty tissue, commonly referred to as lipomas.
How has the medical community responded to the introduction of Tapencarium?
As of now, this groundbreaking drug has undergone evaluation solely through phase 1 and phase 2 FDA clinical trials. The collective sentiment among clinical investigators and scientists, myself included, is one of considerable encouragement, underscoring the noteworthy potency and effectiveness observed in the course of this innovative treatment's development.
How might the widespread adoption of Tapencarium impact the landscape of modern surgical practices?
Adoption will impact the landscape by expanding our ability to perform facial and body contouring fat reduction treatments without having to undergo larger surgical procedures. There is still work to be done including a phase 3 clinical trial, but the future for aesthetics is incredibly bright.
With its revolutionary potential and scientific advancements, Tapencarium stands poised to redefine the contours of aesthetic treatments. As we eagerly await further developments through ongoing clinical trials, the prospect of a transformative impact on modern surgical practices beckons, offering new possibilities in facial and body contouring.
For more information, visit Sachin Shridharani, MD, FACS's social media: