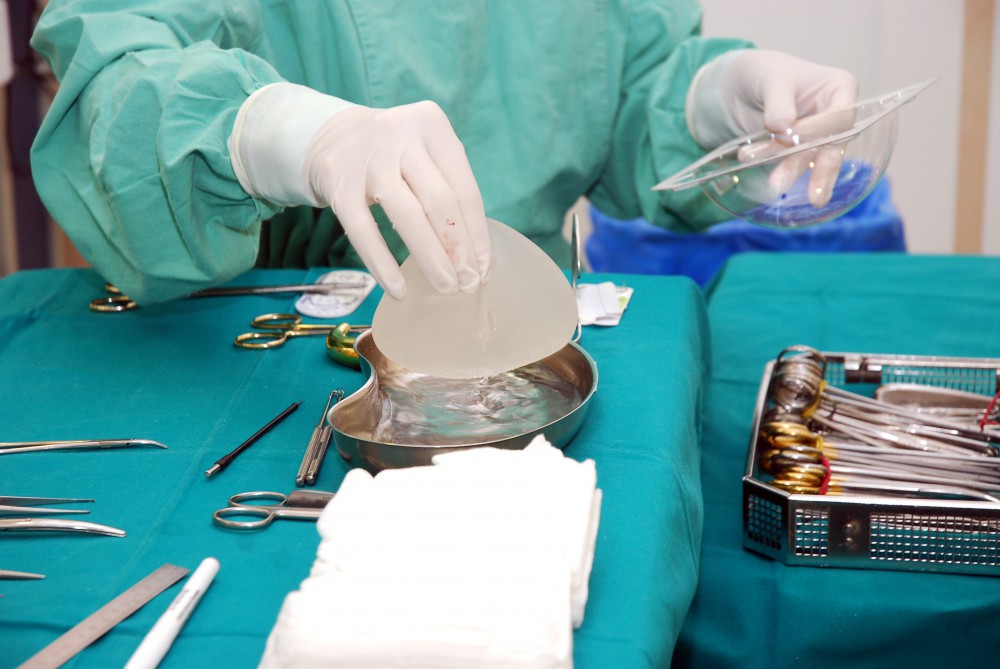
Recently, the FDA administered an advisory panel demanding more information on the risk and benefits of breast implants. The hearing was evoked by a recent surge in patients being diagnosed with specific illnesses related to breast implants. Dr. Aviva Preminger, Breast Expert, sat down with Haute Beauty to discuss the details of the hearing and what future patients should be mindful of when considering this procedure.
What information should our readers know about the latest Breast Implant Safety Hearing?
The hearing was held to discuss the benefits and risks of breast implants for both augmentation and reconstruction purposes, and was prompted by recent coverage of patients being diagnosed with BIA-ALCL or reports of breast-implant illness (BII) and actions by other international regulatory authorities. In addition to plastic surgeons, the panel also heard from breast-implant patients, patient advocates and manufacturers. The FDA will use the panel deliberations to help inform their ongoing goal of ensuring the safety of breast implants.
How will this hearing impact the future of breast implants?
The FDA wants more data and closer follow up before any determinations or recommendations are made. The American Society of Plastics Surgeons has established a breast implant registry to follow patients who have had breast implant surgery. Previously, the FDA enforced a twelve year moratorium (1994 to 2006) on the use of silicone implants to investigate an alleged association with rare connective tissue disorders. There was no cause and effect found and silicone implants were approved for continued use after the investigation concluded.
What is the likelihood of patients becoming ill after breast enhancement surgery?
According to the American Society of Aesthetic Plastic Surgeons, there is no definitive established link between the often subjective and divergent list of breast implant illness symptoms, and no means for testing, there is no ‘known’ risk. Many of the symptoms described by breast implant patients are experienced by the general public on a regular basis with or without implants.
In 1999, The Institute of Medicine Committee on the Safety of Silicone conducted an extensive review of the available literature and concluded there was no demonstrated clear link between silicone implants and any systemic illness. There have been studies of many different sizes and design to look at the safety of breast implants themselves. These have looked at specific autoimmune disorders and diseases. In aggregate, these studies show little to no links between breast implants and any disease. Studies of patients who have symptoms that they have related to their breast implants have not shown consistent laboratory abnormalities to define a distinct syndrome.
To-date, there has been very little in the way of research into this entity that has been labelled Breast Implant Illness (BII). There is no current definitive epidemiological evidence to support a direct link between breast implants and any specific disease process. However, this does not mean further research is not indicated. In rare and unusual disease processes, it can take years to come to a scientific conclusion. There are many factors that can affect the interaction between a patient and her breast implants. Further study is required to determine the best way to potentially screen patients prior to breast implant surgery and to determine which of the multitude of reported symptoms might improve with implant and capsule removal.
A lack of a direct, proven scientific link does not mean that the symptoms experienced by these patients are not real. Some patients have legitimate concerns about a potential link between breast implants and symptoms, so it deserves our attention and further scientific research to better determine what symptoms may improve with explanation of implants.
Patients should, however, be informed of the risks that can be associated with breast implants, including (but not limited to) BIA-ALCL, a rare spectrum of disorders that can range from a benign accumulation of fluids around the breast (seroma) to an extremely rare lymphoma. They should know that BIA-ALCL is not a cancer of the breast tissue itself and that when caught early, it is readily curable. If the disease is advanced, chemotherapy or radiation may be required.
Which patients are more receptive to illness considering this surgery?
The rates of BIA-ALCL seem to be different throughout the world. This may be due to different reporting and registries, but there is likely to be a genetic predisposition that is not yet fully understood. For instance, as of this time there are very few cases in Asian patients. The risk appears to be only with textured implants and not smooth implants; the rate is no different between silicone and saline; it occurs in both cosmetic and reconstructive patients. There is no test to determine whether one textured implant patient is at any more risk of developing this disorder than any other patients.
What safety advice would you give to patients seeking breast enhancements?
Regular follow up. A breast augmentation is never a one-and-done procedure in the sense that annual follow up with a plastic surgeon is recommended.
For more information, visit Dr. Brian A. Levine's social media: