According to the American Society of Plastic Surgeons (ASPS), breast augmentation was the most-performed procedure in 2018 (once again), and almost 314,000 women chose to enhance their appearance with breast implants. “Breast augmentation is consistently among the top cosmetic surgical procedures because of high patient satisfaction rate and relatively easy recovery,” says plastic surgeon Dr. Jose Rodríguez-Feliz of Coral Gables, Florida.
Although the breast augmentation procedure itself has a proven track record of safety, silicone breast implants have had their ups and downs over the past few decades. In 1992, the U.S. FDA placed a moratorium on the use of silicone implants for breast augmentation (with the exception of reconstruction procedures following breast cancer and congenital deformities). Dr. Rodríguez-Feliz explains, “This moratorium was lifted in 2006, and a new generation of breast implants with more cohesive silicone gel became extremely popular—and we’ve seen many additional implant innovations in the years since.”
Then in 2011, the FDA released a report about the connection between textured breast implants and anaplastic large-cell lymphoma (ALCL), a rare type of cancer of the immune system. In 2016, the World Health Organization also recognized that ALCL may develop after a woman receives textured breast implants. Although concrete data about the rate of occurrence is still somewhat limited, studies have suggested that ALCL may develop in one of 3,800 to 30,000 women who get textured implants. “These findings led to textured breast implants being pulled from the European market in December 2018. However, none of the U.S. regulatory agencies examining the role these implants may play in the development of ALCL are recommending that they be removed, and textured implants are still available in the U.S.,” explainsDr. Rodríguez-Feliz.
This past February, a study published in the Aesthetic Surgery Journal revealed that a woman who received buttocks implants was diagnosed with ALCL roughly one year after surgery. If you’re wondering why one case caught the attention of the medical community and the media alike, it’s because buttocks implants have textured outer shells similar to those used in textured breast implants. Dr. Rodríguez-Feliz says, “Of course, plastic surgeons are keeping a close eye on ALCL developments, as this is not something that can be ignored.”
In the latest news, the FDA met last month and was supportive of more comprehensive product labeling and patient consent about the potential risks associated with textured implants, as well as efforts to educate women who already have them. “In the meantime, some prospective breast augmentation patients are contemplating saline implants over silicone implants. Saline implants are filled with sterile salt water and have a smooth outer shell, which means there may be less potential health risks,” says Dr. Rodríguez-Feliz.
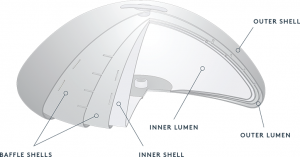
Yet saline implants have been associated with a less natural look and feel than their silicone counterparts in the past. According to Dr. Rodríguez-Feliz, “A new saline implant called the Ideal Implant offers patients the best of both worlds. Designed with two separate chambers of saline, it features an internal support structure that helps minimize the movement of fluid and minimize wrinkling around the outer edge—which are two of the biggest patient complaints about saline. I believe the new Ideal Implant will find a good niche in the current market during this period of uncertainty about the safety of silicone implants, and allow patients to enjoy a new-and-improved breast appearance without the worries associated with silicone.”
For more information, visit Dr. Brian A. Levine's social media: